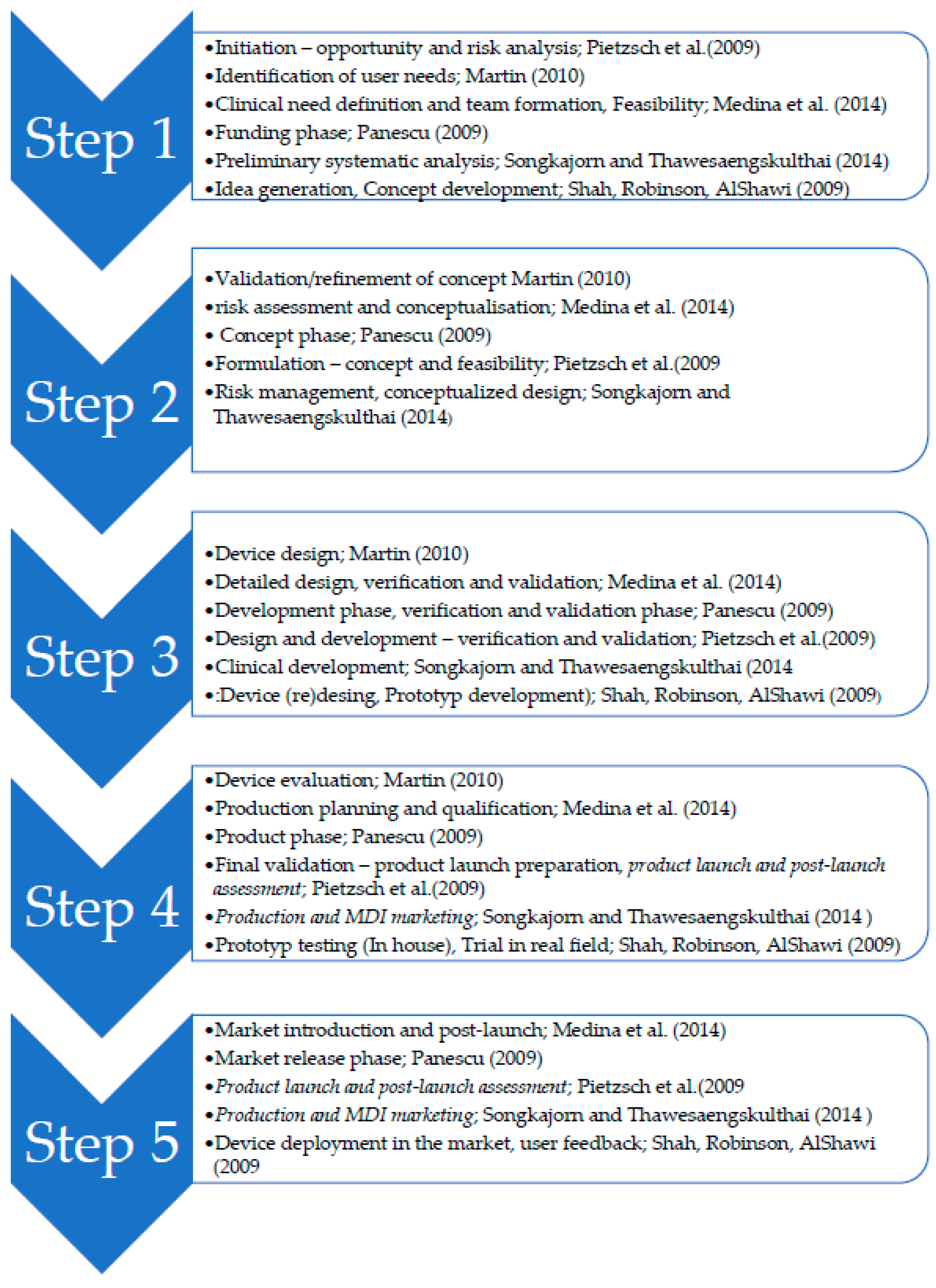
Sustainability | Free Full-Text | Complexity Stage Model of the Medical Device Development Based on Economic Evaluation—MedDee

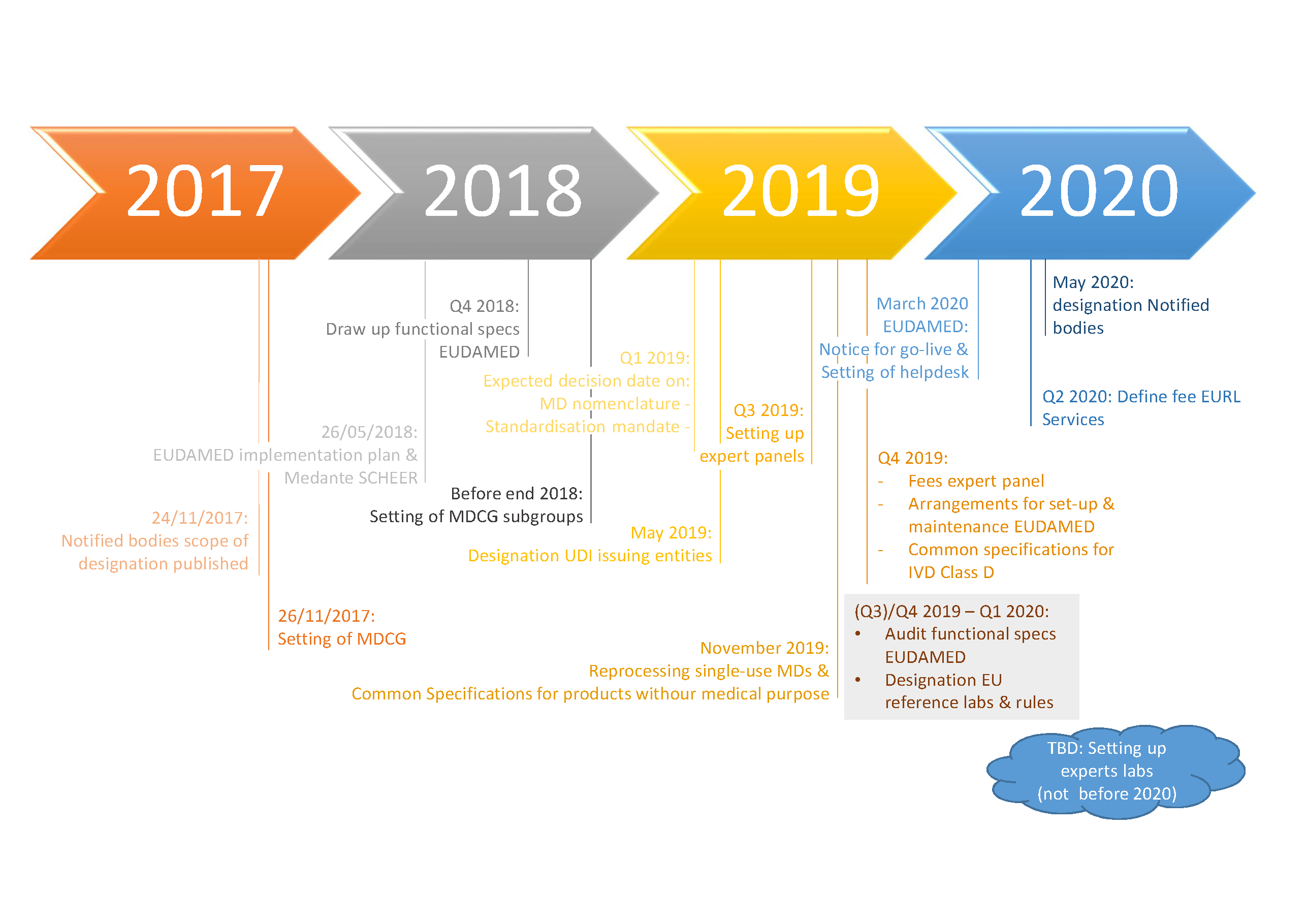
IMPLEMENTATION ROLLING PLAN Regulation (EU) 2017/745 and Regulation (EU) 2017/746. Update dec 2020 - Formiventos